Monoclonal antibody use in asthma is increasing, but not all patients are positioned to reap the benefits.
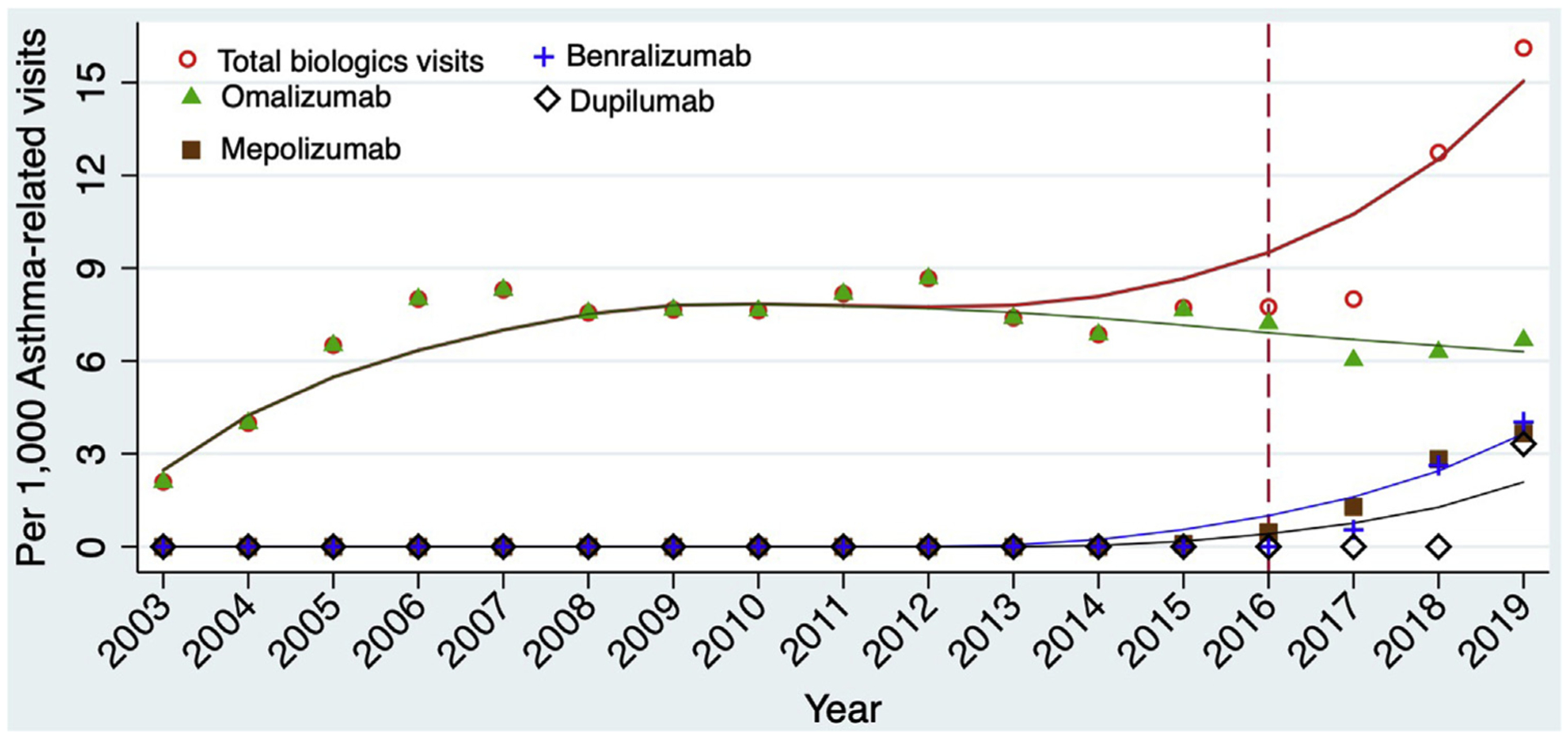
The approval of six biologics over the past two decades has significantly improved the treatment options for patients with asthma and other allergic diseases. However, medications do not work except patients have access to them and they are used. While the number of prescriptions for respiratory biologics in the US continue to increase, patients with public insurance and those belonging to underrepresented racial and ethnic groups lack behind their peers in use. We evaluate why these differences exist and ways in which we can eliminate disparities in the use of novel therapies.
Anti-drug antibodies do occur but their role in the safety or effectiveness of the respiratory biologics is unclear.
Most novel therapies are approved following relatively short efficacy trials. For chronic diseases, like asthma, in which the use of these therapies may be required for much longer periods than in efficacy trials, there are likely to be adverse events that are discovered after approval. The Drug USE Lab is interested in pharmacovigilance related to biologics approved for asthma and other allergic diseases so that the benefits of these therapies are maximized while risks are minimized.
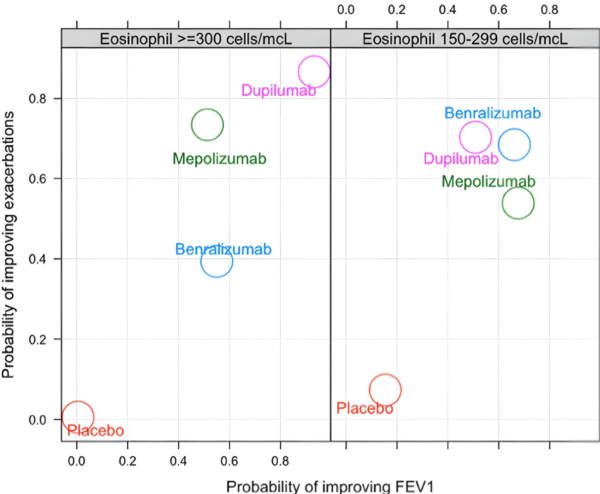
The best biologic depends on the outcome of interest.
Eligibility for the six respiratory monoclonal antibodies “biologics” currently approved for the treatment of asthma is not mutually exclusive. Thus, many patients will meet eligibility criteria for two or more of these therapies. However, there are no head-to-head trials of these biologics making direct comparisons of their effectiveness challenging. Importantly, we have shown that the “most effective” biologic depends on the outcome of interest. For instance, based on our meta-analyses of data from the seminal randomized trials of these biologics, we showed that the respiratory biologic associated with the greatest improvements in lung function in patients with eosinophilic asthma is not the same biologic associated with the greatest improvements in the asthma control questionnaire.
We have used various strategies in generating evidence of the comparative effectiveness of biologics in asthma. These include the use of indirect treatment comparison and network meta-analyses approaches and emulation of hypothetical target trials using data from the electronic health records. We continue to leverage innovative statistical and causal inference methods using advanced confounding adjustment methods to evaluate the comparative benefits of these therapies. In addition, we are interested in the refinement of existing methodologies for comparative effectiveness and the development of new methodologies.
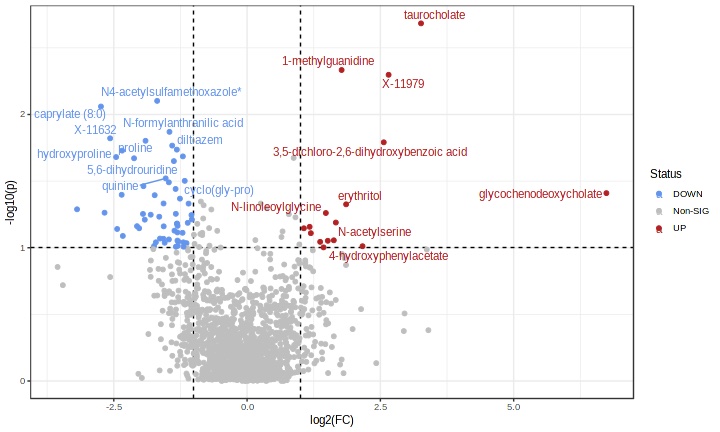
We need more accurate predictors of response to monoclonal antibodies.
The commonly measured biomarkers, such as the peripheral eosinophil count, FeNO, and total serum immunoglobulin E levels, have limited predictive accuracy in differentiating between patients likely to respond optimally to these biologics vs. patients unlikely to. Given asthma-related morbidity and the costs of these therapies, the selection of suboptimal therapy has important ramifications for the patient and the health system. The Drug USE Lab engages in work employing machine learning, predictive modeling, and multi-omics strategies (genomics, proteomics, metabolomics) in identifying predictors of response to these biologics.